Binding Affinity, Gibbs Free Energy, And Intramolecular Energy Explained for Drug Discovery
Written by Amélie Lagacé-O'Connor | Published 2023-7-6
Written by Amélie Lagacé-O'Connor | Published 2023-7-6
In the exciting world of drug discovery, the pursuit to develop life-changing medications depends on understanding the intricate interactions between molecules. Three fundamental concepts lie at the heart of this challenge: binding affinity, Gibbs free energy, and intramolecular energy. These concepts play a vital role in the design and optimization of pharmaceuticals to maximize their effectiveness in combating various diseases and conditions. In this blog post, we will dive into each of these concepts and explore their significance for the development of therapeutic agents.
Binding affinity is all about how strong the interaction is between a drug, (known as the ligand) and its target molecule, typically a protein that plays a critical role in a particular pathway or mechanism related to a disease. Scientists use this value to measure how much the drug is attracted to the binding site on the target, reflecting the likelihood and stability of their binding.
A useful analogy to understand the drug-target interaction is that of the lock and key. In this analogy, the key represents the drug molecule, and the lock represents the target protein, with a binding site in which the drug molecule binds. The strength of the interaction between the drug and its target, known as binding affinity, can be compared to how well the key matches the lock and its ability to unlock it. When a key matches a lock very well, it exhibits a higher binding affinity. This higher affinity results in a stronger and more stable interaction between the drug and its target, ultimately enhancing therapeutic effectiveness. On the other hand, if a key poorly matches the lock, it fails to open it. Similarly, a drug molecule with a lower binding affinity may struggle to engage effectively with its target protein, reducing the likelihood of a complex forming. Consequently, this can lead to a less effective therapeutic agent.
Binding Affinity is affected by many factors such as molecular shape, electrostatic interactions, hydrogen bonding, hydrophobicity, and van der Walls forces. Some techniques used by researchers to manipulate these factors is to make structural modifications, adjusting charge distribution, introducing hydrogen bond donor or acceptor groups, introducing or removing hydrophobic groups, and modifying the spatial arrangement of the ligand in order to optimize binding between the drug and its target and improve drug selectivity. This iterative process of design, synthesis, and evaluation fine-tunes the ligand’s properties and improves its binding affinity to the target molecule.

Gibbs free energy is a thermodynamic concept that measures the energy available to do useful work in a system. In other words, the value represents the energy that the drug ligand needs to bind to the protein complex. A low Gibbs free energy represents an energetically favorable interaction, meaning the two can easily form a stable complex. Conversely, a high Gibbs free energy indicates a weaker or less stable interaction, thus requiring more energy to maintain a complex state.
The change in Gibbs free energy, denoted ΔG, represents the difference in Gibbs free energy between the initial and final state of a reaction or process, giving us insight into its direction and feasibility. A negative value indicates a spontaneous reaction that can occur with no external input of energy. A positive value means the reaction is not spontaneous and therefore requires external energy input to occur.
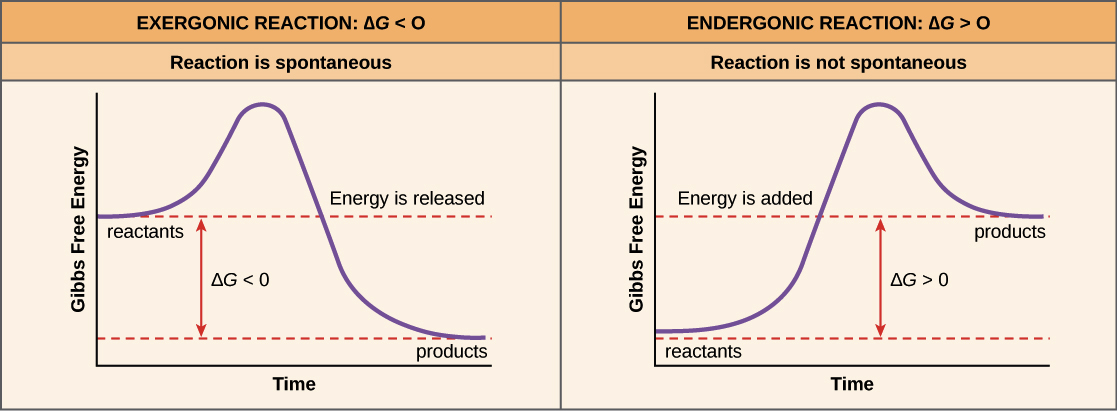
In the context of the previously mentioned lock and key analogy, the Gibbs free energy change represents the requirement of properly aligning the key (drug molecule) within the lock (binding site within the target protein) and overcoming energetic barriers associated with that process.
In the context of drug discovery, Gibbs free energy change during drug-target binding can give information on the likelihood of a drug effectively interacting with its intended target. A favorable change in Gibbs free energy, as previously stated, is typically one that is negative, indicating a strong binding affinity between the drug and its target. This would suggest that the drug has the potential to efficiently exert its therapeutic effects. On the other hand, a positive change in Gibbs free energy would indicate that the binding process between a drug and its target requires the input of energy to occur, indicating a weak binding affinity, therefore suggesting that the drug may exhibit weaker therapeutic effects.
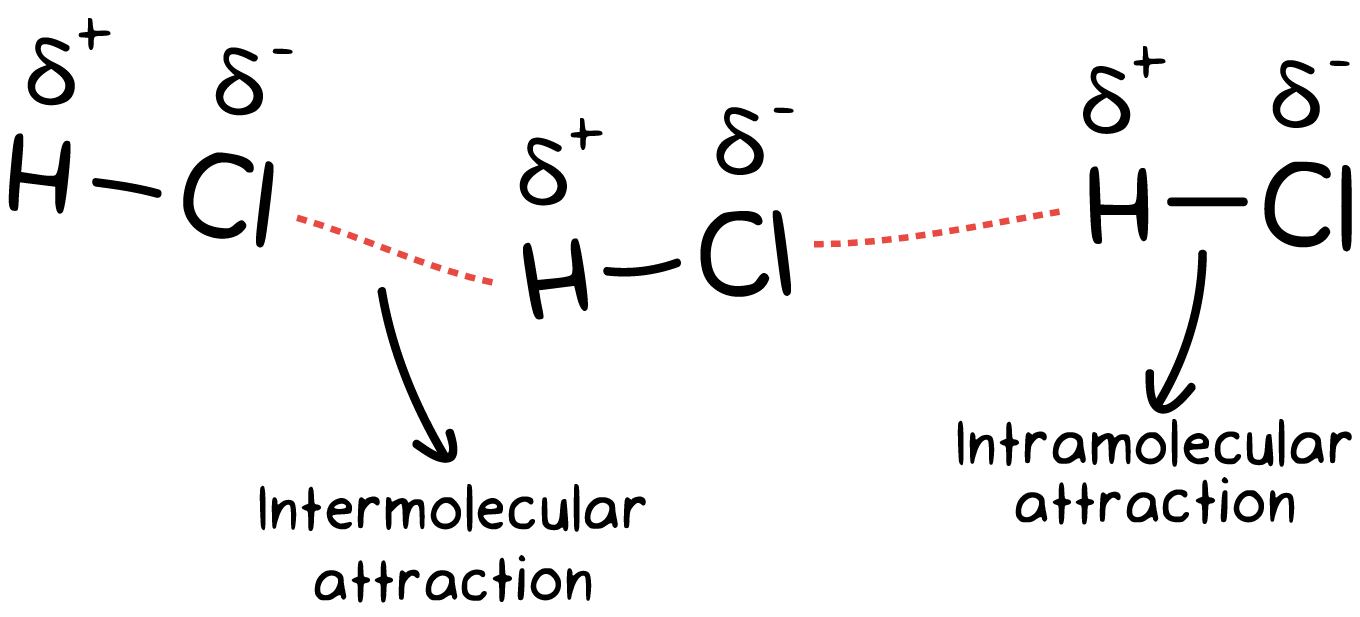
Intramolecular energy refers to the energy contained within a single molecule, holding the bonds together. The value represents the energy that is necessary to break the bonds within the molecule. A high intramolecular energy indicates stronger bonds, while a lower value indicates weaker bonds. It plays a key role in drug design by influencing the molecule’s conformational changes, flexibility, and adaptability to target binding sites.
In the context of the lock and key analogy, the intramolecular energy refers to only an imaginary dynamic key with internal moving parts. It would represent the ability the key has to adopt different shapes and groves, which would be crucial for it to properly fit into a specific lock. A high intramolecular energy would make it harder for the key to adopt different structures, compared to a lower intramolecular energy.
Relating this back to a drug molecule, one containing a high intramolecular energy means that it would not as easily be able to change conformations to fit into the shape of the binding site found within its target protein. Therefore, to optimize a drug’s therapeutic capabilities, having a lower intramolecular energy would allow it to change conformations more easily, enabling it to bind to its target protein and form a complex.
Understanding and analyzing intramolecular energy through tools such as molecular dynamics simulations and quantum mechanics calculations, allow for the optimization of drug molecules. Researchers exploit this characteristic and design drugs with enhanced potency and improved target engagement and efficiency.
In summary, binding affinity, Gibbs free energy, and intramolecular energy are interconnected and each play a role in designing and optimizing drug molecules and their interactions with target molecules.
Binding affinity refers to the strength of the interaction between a drug and its target protein. Gibbs free energy measures the stability and spontaneity of the drug-target complex, where the change in Gibbs free energy represents the associated energy change during the formation of the complex. The intramolecular energy measures the strength of the bonds within the drug molecule and indicates its ability to change shapes, thereby complementing the binding site of its target protein.
By understanding and manipulating these factors, researchers and biotechnology companies can advance the discovery and design of innovative therapeutic agents for various diseases and conditions.
If you’re interested in calculating the Binding Affinity, Gibbs Free Energy, And Intramolecular Energy of a protein-ligand pair, try Neurosnap’s DiffDock service which calculates all these metrics and more!
By Danial Gharaie Amirabadi
By Keaun Amani
By Keaun Amani
By Keaun Amani
By Keaun Amani
By Danial Gharaie Amirabadi
Register for free — upgrade anytime.
Interested in getting a license? Contact Sales.
Sign up free